Parsley for instance is known as prezzemolo in standard Italian, but I grew up calling it petrosino. Interestingly parsley's genus, derived from Latin, is Petroselinum. The modern Italian word also originates from Petroselinum, but obviously the dialect-version of parsley is closer to its roots, pardon the pun. The English word seems to have been influenced by the Old French peresil and by the old English petersilie, which in turn is an offshoot of the Latin.
The Greeks once referred to the herb as petroselinon, which meant "rock celery", but the modern Greek word is based on the Turkish word maydanoz.
Petroselinum latifolium photo by Forest and Kim Starr

Now that we know parsley's etymology, we can investigate its chemistry. Simple distillation equipment may be fine for creating moonshine, but it will not do much to separate the compounds of parsley or of any plant for that matter. What's needed is the Likens Nickerson Method. The parsley-water mixture is placed in one flask, and a separate flask holds an organic solvent. Both are boiled while being connected to a shared set of columns and condenser. Using dry ice, water is removed from the fractions, and eventually the compounds are separated and identified through good-old gas chromatography-mass spectrometry.
The main volatile component of oils in parsley is apiole (also spelled apiol), C12H14O4 .
All sorts of medicinal properties have been attributed to apiole since the mid-19th century, but in concentrated form it can do a number on your kidneys and liver.
The apiole molecule bears a strong resemblance to another substance found in small quantities in parsley: myristicin.
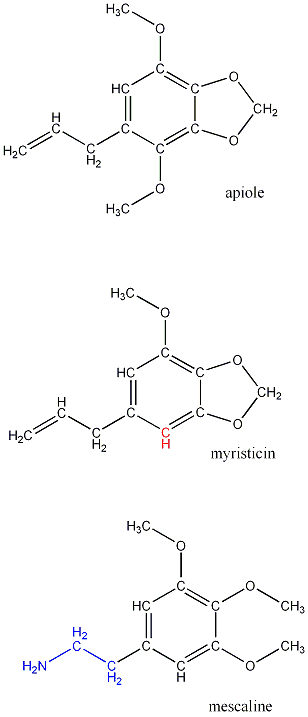
Myristicin is the notorious alkaloid drug which is also found in nutmeg. In the latter, it is found in a greater percentage and coexists with other natural drugs. In the 1970's pursuit of altered states inspired many inmates to ingest spoonfuls of the spice. Possible side-reactions include chemotherapy-like vomiting and possibly death, a somewhat high price for hallucinations.
As we compare the structure of parsley's non-psychoactive apiole to that of myristicin, we notice that one of the methoxy(O-CH3)groups is also replaced with hydrogen.
As is often pointed out in the literature, myrisiticin, although not an alkaloid, is structurally very similar to peyote's hallucinogen, mescaline. Rather than being part of separate methoxy groups, the oxygens in myristicin form a heterocyclic ring, and in mescaline, the allylic group is in a reduced state and the tail-end carbon is replaced with an amino group.
The similarities in natural products are not coincidences. In their bio-synthesis, they share a common pathway. Side-reactions then occur for various reasons, and they are subject to natural selection.
Molecules, like words, often have common roots, but they are in a constant state of flux.
Sources
- http://www.uni-graz.at/~katzer/engl/Petr_cri.html
- The Merck Index. Twelfth Edition
- Pol. J. Food Nutr. Sci. 2005, Vol. 14/55,No 1, pp. 63–66